The Neuroscience of Method Teaming – an Independent Review

The Method Teaming instrument set, which spans behaviors, motivators and cognitive processes, provides the most comprehensive talent scan available in the world today. Psychological theory clearly endorses its validity whilst 20 years on-the-ground experience of the application of Method Teaming has shown that it has no peer in matching individuals to the right role in a team or organization.
But what does the world of neuroscience have to say on the subject? After all, personality is, at least principally, grounded in neural processes. Does neuroscience confirm or deny that Method Teaming provides comprehensive coverage of the human brain’s workings as they relate to the work environment?
OND LLC engaged Alex Nagle, a neuroscience Masters graduate (Distinction) to conduct a review and find the answer. Alex’s research took 3 months and involved a journey through nearly 90 academic papers and writings. Breaking new ground with his work, Alex discovered that the Method Teaming instruments connect with an astonishingly high proportion of the regions and circuits of the brain that are involved with the higher order thinking processes involved in work. His detailed findings, naming and labelling each of these regions and circuits, revealed that the breadth and reach of Method Teaming is as close to full coverage as can be. Truly it can be said that Method Teaming leaves no significant area of the brain untouched as it scans the cerebral landscape for every person’s natural strengths and talents.
Here is Alex Nagle’s full review. To download this review in pdf click: Neural Correlates of Method Teaming by Alex Nagle
The Neural Correlates of Method Teaming.
Alex Nagle, MSc
Abstract. Since its conception, OND has grappled to dissect the complexities of intellectual diversity amongst individuals. With such knowledge at the disposal of a business, employees can be stationed to the roles that match their innate talents, thus augmenting team efficiency and satisfaction. After a thorough assessment of the psychological research, OND has collated the most precise personality trait instruments into a single holistic assessment of personality for business use – Method Teaming. Whilst the psychological theory clearly endorses its validity, how does it stand up to the most recent neuroscientific theory? Personality is, at least principally, grounded in neural processes, and thus neuroscience research is the ultimate authority for its authentication. This assessment will let the science do the talking as you discover the neural correlates of Method Teaming and the power of individuals.
1. Introduction
Personality is an elusive phenomenon in the neuroscientific realm, hitherto unyielding to the classical reductionist approaches of empirical investigation. Perhaps part of its mystery owes to our apparent struggle to first characterise it conceptually. For how can one interrogate something meaningfully when it lacks a conceptual definition? The definition need not wholly match the truth, but it must first exist in order for its credibility to be substantiated or invalidated by research. And yet, how does one define something when its very essence appears to transcend language and its complexity perforates the limits of our intellectual capacity as humans? This is the enigmatic and impervious territory in which personality still in part resides, blanketed in a thick sludge through which even the staunchest academic explorers ultimately lose traction and stagnate.
A melodramatic description, you must be thinking, and a pessimistic opening to a review which claims to map personality traits, as defined by OND’s comprehensive Method Teaming tool, onto their physiological correlates within the brain. I simply wish to convey from the onset that this field is still infantile in its sophistication, and cannot yet boast discovery of an unambiguous, unified neuroscientific model of personality. At this stage, a more methodologically feasible approach is to tackle personality trait-by-trait, dismantling it into more bite-sized, manageable chunks for analysis. Whilst some holistic qualities of personality may be lost within this reductionist perspective, it is nonetheless a rational method. Indeed, advances in quantitative psychology have, within the last few decades, attempted to identify the primary traits of personality and reliably measure their variation between individuals. This is where OND’s Method Teaming tool is so powerful. They have carefully collated three of these renowned psychological assessments – DiSC, PIAV and HVP – into a single, more robust, test of personality that provides an encyclopaedic insight into an individual’s trait variation. From this, OND can reliably inform businesses of the weaknesses in their teams’ structure, and work towards assigning roles to match peoples’ true intellects, thus bolstering corporate efficiency, as well as individual wellbeing.
Method Teaming is therefore uniquely poised to cater maximally to team productivity; it evaluates personality from three distinct angles of behaviour (DiSC), motivation (PIAV) and cognitive structure (HVP). As will be seen, and especially because of HVP, this delivers a thoroughness lacking in other measuring systems that focus on only one or two aspects of personality and thereby interrogate fewer regions of the brain. Indeed, a recurring dogma from the psychological literature agrees that personality traits are “probabilistic descriptions of relatively stable patterns of” motivation, cognition and affect (emotion), as they lead to behaviour (DeYoung, 2015). To place these traits in the context of our day-to-day lives, they likely enable an individual to identify goals (cognition), to be impelled to achieve those goals (motivation), to select and execute suitable actions to move toward them (behaviour), reflect on the impact of those actions on themselves and the world (cognition), and detect whether their current state matches their goal state (affect; Widiger, 2017). This is the compelling, albeit ultimately under-sophisticated, model of personality as seen through the lens of cybernetics – the study of goal-directed, self-regulating systems (Carver and Scheier, 1998; Wiener, 1961) that is a “useful and perhaps even necessary approach for understanding living systems” (Gray, 2004). Immediately, one can observe how the Method Teaming instruments neatly align with the cybernetic perspective of personality. The initial identification of goals reflects the HPV (cognitive structure) instrument, which shines a light on the value system of each individual. It is this value system which likely provides the framework under which the individual will establish goals that seem appropriate to him or her. The PIAV instrument exemplifies the intrinsic motivators that guide the individual toward the goals, be it aesthetic appreciation, social valuation, theoretical discovery, or something else. DiSC then assesses how the individual is likely to manifest all these inner theorisations overtly in the world in terms of behaviours. Whilst the idea of ‘affect’ is not directly mirrored within Method Teaming, it is arguably not as relevant to businesses. Yet perhaps this view will change in the coming years. Nonetheless, from a psychological perspective at least, Method Teaming closely emulates the most recent psychological theories and therefore provides a comprehensive assessment of personality for business use. However, a story that remains untold is how Method Teaming’s three instruments map onto the physicalist domain. What are the neural correlates of Method Teaming? Does Method Teaming provide a similarly complete picture of the brain’s functioning (as we understand it now) as it does with current psychological models?
A common misconception is that the entire brain must be implicated in manifesting an individual’s personality. This is likely not the case. A crude, but useful, approach for dividing the brain based on anatomical and functional characteristics is through the cerebrum-cerebellum-brainstem trichotomy (a model that will be referred to throughout this review; Bear et al., 2020; Figure 1). The cerebrum is the largest part that we popularly associate with ‘the brain’ – that pinkish, deeply wrinkled ball comprising two hemispheres that together regulate the myriad higher order functions such as reasoning, learning, sensory perception and emotion, to name but a few (Bear et al., 2020). The cerebellum lies underneath the cerebrum and towards the posterior. It is responsible for coordinating and finetuning voluntary muscle movements. For its size, this region contains a disproportionately large number of neurons – even more than the cerebrum – which is testament to the complex requirements of motor control (Bear et al., 2020). Lastly, the brainstem is located anterior to the cerebellum, still below the cerebrum, and governs relatively primitive processes such as breathing, cardiac function, and reflex behaviours (Bear et al., 2020). From these brief descriptions, one can infer that the brainstem and cerebellum are likely minimally involved in manifesting personality traits, in contrast to the cerebrum which dictates the majority of higher order executive processes. Therefore, it is no failure of Method Teaming if it is shown not to correspond to every brain region exhaustively. A more realistic outcome is to demonstrate that the three instruments of Method Teaming, whilst partially overlapping in their trait evaluation capabilities, ultimately cater for distinct elements of personality and map to different parts of the brain, thus painting a more polished picture of an individual’s psyche when combined.
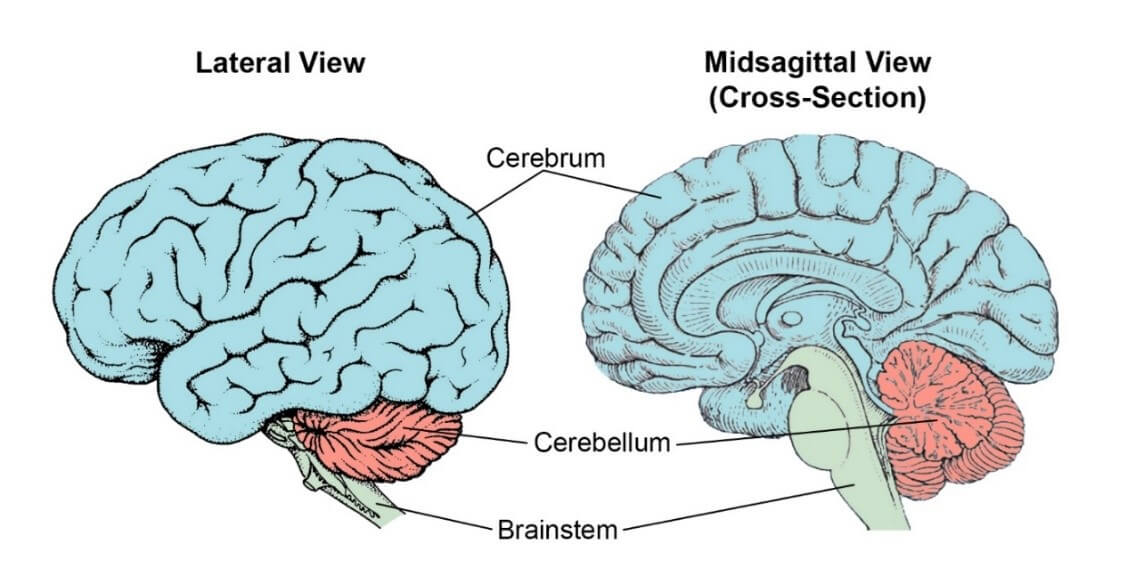
Figure 1. The cerebrum-cerebellum-brainstem anatomical stratification of the brain. The brain can be crudely separated into three regions based on spatial and functional characteristics. The figure depicts two views of the brain; on the left, the brain as a whole is viewed from the side (lateral); on the right, the brain is viewed as if cross-sectioned down the middle from front to back (midsagittal). The cerebrum (blue) is the large bulbous region that is commonly associated with ‘the brain’. It contains regions that are essential for regulating complex cognition processes, emotion, vision, sensory perception and an unthinkably vast array of other functions. The cerebellum (red) lies just beneath and towards the back. It is even more deeply folded and wrinkled than the cerebrum, in order to more efficiently pack the large number of neurons that subserve it. The cerebellum is primarily charged with regulating voluntary movement and for finetuning motor processes as they occur. The brainstem (green) lies at the bottom of the brain but protrudes up into the cerebrum, and is the mediator between the spinal cord and the rest of the central nervous system. Principally, it governs automatic physiological processes such as breathing, cardiac function and reflexes. There are other ways in which the brain can be stratified into different regions, but the cerebrum-cerebellum-brainstem trichotomy seems most comprehensible and instructive for the purposes of this review.
Within this review, each instrument of Method Teaming will be tackled in turn, and their physiological basis within the brain will be discussed in light of the most recent literature. However, it is important to stress that this review should not be read as an infallible account of the truth, but rather as an inchoate draft that does well to capture some basic principles but ultimately undershoots the exact truth to some degree; science, for all its worth, is a work in progress and its data should be perpetually held to account, especially for a complex matter such as this. For instance, many studies in personality neuroscience are correlational by nature (e.g. Adelstein et al., 2011), and it does not take a scientist to remember that ‘correlation does not imply causation’, and thus appreciate that such studies possess an intrinsic limitation to their interpretation. This ‘correlational handicap’ is present because personality neuroscience studies must often rely on human models (e.g. Markett et al., 2018), seeing as personality (as we see it manifest in individuals) is arguably an exclusively human phenomenon. In animal models, ethical sanctions allow for invasive techniques to reveal causal relationships. In humans, however, whilst investigations of neural activity are not inconceivable (using non-invasive electroencephalography, for instance), living human brains simply cannot be subject to the same range of manipulations to reveal causal patterns. This is one factor amongst many that has recently led to a ‘replication crisis’ in psychology and threatened the credibility of numerous published studies (Lilienfeld, 2017). Nonetheless, rigorous and honest scientific research is an incredibly potent tool. On the unanimous basis that personality is, at least principally, derived from neural processes, there is no doubt that neurobiological investigation will yield some important findings for elucidating personality. It is likely that such results will eventually translate into the field of psychometrics and augment the sophistication and accuracy of personality assessments such as Method Teaming. However, it is improbable that such biological measures will wholly usurp psychological and self-report tests. Personality is inherently a hybrid discipline, and it would be imprudent to divorce it from its phenomenological and qualitative aspects that clearly hold a unique and irreplaceable niche for describing it. Not to mention, who wants to reduce an individual’s most fundamental essence to a sterile series of molecules and numbers? Before I slip further into a hole of philosophical musings, we may now leave behind this long-winded, yet mandatory, prologue and engage with the issue at hand: to discuss the neural correlates of personality and demonstrate that there is method to Method Teaming.
2. DiSC.
DiSC is a long-established psychological assay of behaviour. It is designed to assess behaviour as it manifests inside a group. Combined with a relatively simple and comprehensible output, DiSC therefore lends itself nicely to implementation in the world of work by HR managers, consultants, personal coaches, and the like (Reynierse et al., 2000; Diekmann and Konig, 2015). Additionally, behaviour is a composite of surface traits – traits that are observable and thus can be measured with good reliability (Johnson, 2019). Consequently, behavioural assessments, when constructed carefully, can often boast a higher level of accuracy than that seen in motivational, cognitive or emotional assessments, in which their respective source traits are not directly observable and must instead be inferred.
Through a self-report assay, DiSC stratifies individuals by their conformity to four distinct behavioural groups. The Dominant ‘D’ group describes outgoing, task-oriented individuals that are intent on completing tasks and getting to the crux of an issue quickly. They may also be described as ‘driving’ or ‘doer’. The Inspiring ‘I’ group pertains to those individuals that are outgoing and people-oriented. They enjoy interaction, socialising and having fun, and will be very perceptive to the judgements of other people. The words ‘interesting’ and ‘interactive’ are also used to describe them. The Supportive ‘S’ group encompasses reserved and people-oriented individuals who relish relationships, aiding others and working collaboratively. Their auxiliary adjectives are ‘steady’ and ‘stable’. Lastly, the Cautious ‘C’ group includes individuals who are reserved and task-oriented. They value consistency and quality information, and adhere to being correct and accurate in what they do. They may also be described as ‘competent’ and ‘careful’. This four-factor model has withstood decades of competing theories and has been widely endorsed for its comprehensiveness and utility for team analysis. Indeed, one prominent study has demonstrated that a balancing of DiSC types within teams leads to better outcomes (Lykourentzou et al., 2016).
Whilst DiSC’s psychological underpinnings have been robustly tested, how does it translate to the physicalist domain of the central nervous system? What are the neural correlates of DiSC? Firstly, it is important to state at this juncture that ‘personality’ is a term that has been, understandably, butchered and subject to a host of different definitional systems across time. For this reason, in each instance of its use, such as in this paper, its definition, as construed by the author, must be stated clearly early on. Indeed, I gave a shortened version of the compelling definition from DeYoung (2015). However, even now amidst such intelligent descriptions, there remains in the literature a smorgasbord of varying and unstandardised terminology for personality. Whilst this is to be expected and, to some degree, encouraged (personality is immensely complex and we must not so quickly settle on a single definition), it poses a great challenge to those who wish to assess the literature and collate its isolated stories into a single cohesive narrative. For instance, the adjectives used for the four behavioural traits in DiSC – dominant, inspiring, supportive and cautious – are rarely mirrored in the neuroscientific landscape; the vocabulary of psychological theory does not consistently translate to neuroscientific theory. One exception could be ‘The Big Five’ – a relatively recent and successful model of personality (John et al., 1991), born under DeYoung’s same definitional framework (DeYoung, 2015), that has also been adopted by neuroscientists in an attempt to pursue a more interdisciplinary, integrative personality theory. This is exciting work, but is objectively less applicable to teams and the business world, given its sole emphasis on the individual rather than group dynamics. To return from this minor digression, often the exact vocabulary used in Method Teaming’s three instruments are not present in the corresponding neuroscientific literature. Thus, I do my best to bridge the lexical discrepancies but perhaps some relevant studies slip past my net.
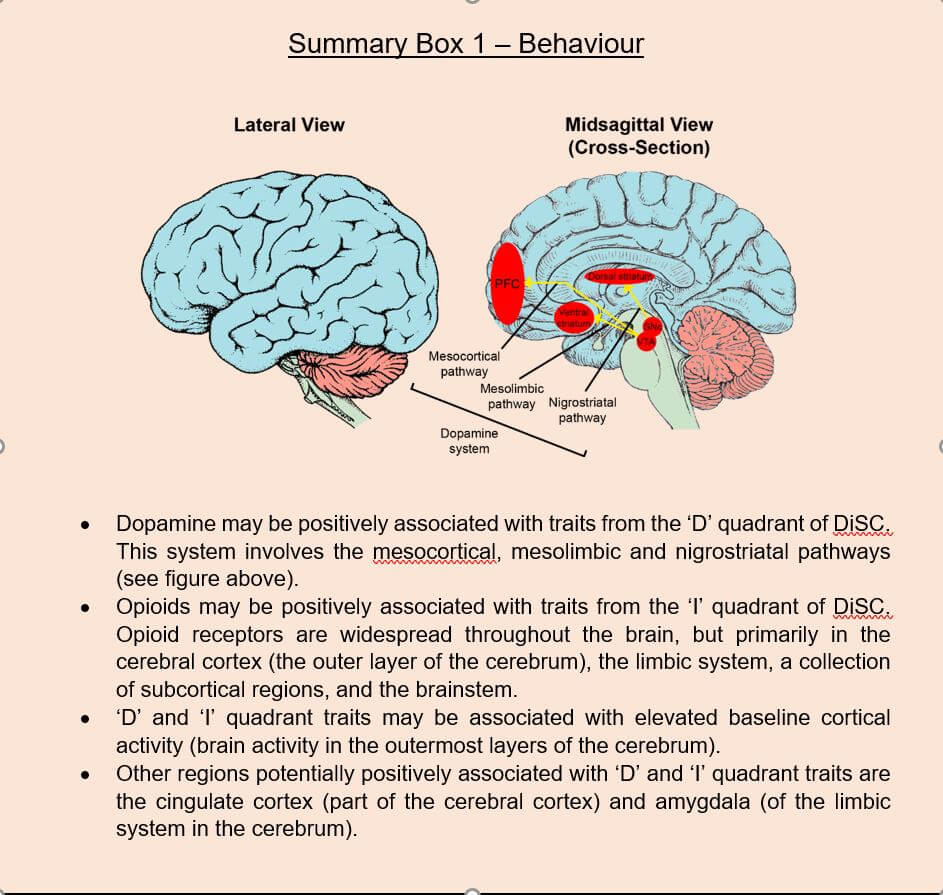
‘Extraversion’ is a personality trait that has been subject to neuroscientific analysis. It represents positive emotionality, enthusiasm, assertiveness and sociability (Wilt and Revelle, 2017). These adjectives are clearly connected to the ‘outgoing’ hemisphere of the DiSC model that encompasses both the ‘D’ and ‘I’ groups. A now widely accepted neural correlate of extraversion is the reward processing system, a discovery that can be traced back to the pioneering work of Jeffrey Gray (1973) and Depue and Collins (1999). Reward circuitry governs our “affective reactions to received rewards, behavioural efforts expended to obtain rewards, and neural activity during reward evaluation” (Smillie, 2013). Both Gray and Depue’s labs posited an involvement of the neurotransmitter dopamine in reward signalling, a theory that has been heavily substantiated (DeYoung, 2010; Smillie, 2008) and further popularised in the non-scientific community. The dopaminergic circuitry is sprawled across the central nervous system and innervates a large number of brain regions. The most prominent dopaminergic circuits for reward signalling include the mesolimbic, mesocortical and nigrostriatal pathways (Luo and Huang, 2016). The mesolimbic pathway originates in the ventral tegmental area (VTA) of the midbrain (part of the brainstem) and projects to the nucleus accumbens and olfactory tubercle of the ventral striatum (part of the basal ganglia which lies deep within the cerebrum; Ikemoto, 2010). The mesocortical pathway also originates in the VTA, but instead innervates the prefrontal cortex (PFC), the anterior surface of the cerebrum (Ikemoto, 2010). Lastly, the nigrostriatal pathway transmits dopamine from the substantia nigra pars compacta (SNc) and innervates the caudate and putamen within the dorsal striatum (part of the basal ganglia; Luo and Huang, 2016). To demonstrate a strong positive relationship of the dopaminergic system with trait extraversion, one previous seminal study sought to measure an electroencephalographic (EEG) signature of dopamine activity, called feedback-related negativity, in human introvert and extravert subjects (Smillie et al., 2011). Feedback-related negativity is an index of dopamine activity present during a reward-prediction error (Potts et al., 2006; Eppinger et al., 2008) – when an unexpected reward occurs or an expected reward does not occur. The researchers found that the intensity of the feedback-related negativity measurement varied consistently between introverts and extraverts, where extraverts demonstrated greater feedback-related negativity signatures. This therefore suggests dopaminergic activity to be a strong correlative hallmark of extraversion, although conflicting results from other studies do exist.
This inconsistency of findings may result from an understated distinction within the brain’s reward circuitry that pertains to the desire for reward versus the enjoyment of reward (Smillie, 2013). Psychologists have also made similar observations that extraversion in fact consists of two slightly different aspects, namely assertiveness and enthusiasm (DeYoung, 2015). Assertiveness is thought to describe achievement-related and motivational behaviour, whereas enthusiasm has more affiliative connotations and describes the enjoyment of social closeness and engagement. This divergence of (more task-oriented) assertiveness and (more people-oriented) enthusiasm neatly aligns with the two quadrants from the outgoing hemisphere of DiSC, the dominant ‘D’ group and the inspiring ‘I’ group, respectively. In the brain, the psychological ‘D’ and ‘I’ distinction is mirrored in the reward circuitry. The dopaminergic system is thought to be implicated in the pre-attainment desire for reward and the assertive aspect of extraversion (the ‘D’ group), whereas the forebrain opioid system is responsible for processing the enjoyment of attained reward and the enthusiasm aspect of extraversion (the ‘I’ group; Knutson and Bhanji, 2006; Berridge et al., 2009). This distinction likely accounts for some of the contradictory findings between studies of extraversion, who likely categorised extraversion in a single broad-brush stroke, and did not account for its bidimensionality of assertiveness (‘D’) and enthusiasm (‘I’). Thus, the neural correlates of the ‘D’ group are likely heavily embedded in the dopaminergic system, whilst those of the ‘I’ group have more of an opioid system emphasis. This is, of course, an immense simplification, and does not account for the inevitable cross-talk and interplay between other circuits, but nonetheless appears to be an evidenced elementary foundation to build upon further.
A separate, not necessarily mutually exclusive, neurobiological motif of the extraversion-introversion spectrum is an extrapolation from Hans Eysenck’s theory: introverts exhibit higher levels of basal brain activity and cortical arousal than extraverts (Eysenck, 1967). At first, this might seem counterintuitive, given that extraverts appear to ‘do more’, as though this was the manifestation of a heightened baseline of neural activity. However, it appears that the opposite is true, and the hypothesis posits that the consequence of this chronic hypo-arousal in extraverts is an increased susceptibility to boredom (Eysenck, 1982). This, in turn, fosters behaviours that seek to quench the boredom such as engaging in stimulating and sociable activities. Indeed, EEG studies have consistently shown dampened basal cortical signatures in extraverted individuals (Matthews and Amelang, 1993; Baumgartl et al., 2020). Therefore, is it possible that the more sensitive and prevalent reward circuitry observed in extraverts is in fact an artefact – a consequence – of their lower basal brain activity? Does the chronic hypo-arousal necessitate a more dominant dopaminergic and opioid system to combat the rapid environmental habituation so that it does not become pathological? Perhaps this is an avenue for further research, to bridge the gaps between these parallel perspectives on extraversion and how they interrelate, if they even co-exist at all.
Another pervasive neuroscientific tool for elucidating personality is functional magnetic resonance imaging (fMRI). This neuroimaging device has become the golden child of modern-day cognitive neuroscience. The non-invasiveness, accessibility and considerable spatial resolution of this method has led to its commonplace involvement in research (albeit at a steep price; Logothetis, 2008). Several relatively recent fMRI studies have proposed a number of various regions believed to be implicated in extraversion (Lei et al., 2015). Recurring regions flagged by these studies include the cingulate cortex, PFC, nucleus accumbens (NucAcc), and amygdala (Kumari et al., 2004; Eisenberger et al., 2005; Suslow et al., 2010). This data appears to conform with the aforementioned ‘reward circuitry’ and ‘cortical activity’ models. For instance, the cingulate cortex and PFC are, as their names suggest, localised to the cortex of the cerebrum, and therefore likely contribute to the differential surface EEG signatures observed between introverted and extraverted subjects. Additionally, both of these regions, along with the NucAcc and amygdala, are established consignees of reward signalling pathways (Luo and Huang, 2016), and are thus consistent with a reward-based theory of extraversion. The amygdala is a famous (or rather, infamous) hub of connectivity located in the limbic system of the cerebrum. It is heavily involved in regulating the approach / avoidance behaviours (Miller et al., 2019) that so clearly seem to differentiate extraverts from introverts, as well as the affective aspects of this personality trait.
There is a clear positive correlation between the degree of extraversion and positive emotionality, as well as a negative correlation with anxiety and major depressive disorder (Yamasue et al., 2008). However, it is important to reiterate extraversion-introversion as a primarily behavioural classifier, not an affective one. For instance, as Smillie (2013) writes, “there is no a priori reason to expect these patterns of affect and behaviour ‘go together’ in terms of their joint covariation across individuals.” However, one can see how a greater proclivity to engage in stimulating, outgoing and rewarding activities will yield more frequent or more intense experiences of positive emotion. Thus, the affective aspect of extraversion is likely an artefact of its core processes, and is not causative. It is also somewhat intuitive to see, on the other hand, how introverts may be more susceptible to experiencing negative emotion. The apparent higher level of basal neural activity in introverts, as discussed previously, is consistent with established theories that describe anxiety as a manifestation of aberrantly excitable and hyper-communicative neuronal signalling (Duval et al., 2015). Indeed, the neurotransmitter GABA, which acts almost universally to inhibit neuronal activity, is heavily implicated in models of pathological anxiety, where it is seen to be deficient (Nuss, 2015).
Through this discussion, I have seemingly not referred much to the opposing hemisphere of DiSC that comprises the ‘introverted’ ‘S’ and ‘C’ quadrants. However, extraversion is considered one end of a behavioural spectrum, with introversion at the other. As a result, those discoveries about extraversion can likely be flipped upside down and applied to the ‘supportive’ and ‘cautious’ groups of DiSC.
These behavioural categorisations are clearly heavily supported in the psychological literature as universal classifiers of behavioural personality. Moreover, they have, thus far, proven to map differentially to different regions of the brain, albeit predominantly in the cerebrum. However, the midbrain region of the brainstem appears to be a major initiator of dopamine signalling, and therefore is perhaps more heavily involved in personality than originally expected. Yet, arguably, the behavioural aspect of personality represents the most primitive and evolutionarily conserved mechanisms compared to the other personality elements of motivation and cognition. Therefore, we might anticipate a recession of midbrain involvement as we progress onwards to the two other personality instruments.
3. PIAV – The Motivational Element of Personality.
Simply put, motivations describe the reasons that people do what they do. I wish I could leave it there, but as I’m sure you are now aware if you weren’t already, the scientific perspective bestows several further layers of complexity. One notable conceptual stratification of motivation seen in the psychological literature relates to innate versus extrinsic versus intrinsic motivation (Di Domenico and Ryan, 2017; Reeve and Lee, 2019). Innate motivation concerns those basal biological desires such as hunger, thirst, sleep or sex that are so highly conserved across animal species (Reeve and Lee, 2019). Whilst crucial to our survival, these are relatively acute motivational states that arguably do not contribute so much to personality traits, that are stable and persistent in comparison. Such innate motivations are unanimously thought to localise to the homeostatic circuits of the brain, namely the hypothalamus in the cerebrum, and the dopaminergic circuits, namely the mesolimbic pathway (Saper et al., 2002). Extrinsic motivation pertains to slightly more sophisticated states – “those learned by the rewarding properties of environmental stimuli” (Reeve and Lee, 2019). By pursuing an extrinsic impetus, an individual is hoping to achieve an instrumentally separable consequence, such as reward attainment or punishment avoidance. Intrinsic motivation, on the other hand, relates to when an individual spontaneously engages with an activity because they find it inherently satisfying and can derive enjoyment and interest from it. For instance, one may be driven to study for an exam because of the instrumentally separable consequence of achieving good grades (extrinsic motivation), or because of the gratification that comes with being intellectually engrossed and learning new material (intrinsic motivation). Importantly, the relationship between extrinsic and intrinsic motivation is not synergistic, as might be expected (Atkinson, 1964; Porter and Lawler, 1968). In other words, when an external reward is dispensed to an individual engaging in an intrinsically motivating activity, the effects on the level of motivation are not additive. Instead, such tangible rewards, that are the consummatory endpoint of extrinsically motivated activities, instead undermine the magnitude of enjoyment and engagement experienced when intrinsically motivated (Deci, 1971; Deci et al., 1999). Perhaps this phenomenon is a contributing factor to the ever-increasing incidence of mental health disorders; in a world where ephemeral rewards that play on cheap impulses have saturated our culture, from social media to online shopping to indulgent saccharine snacks, our capacity to engage deeply with ourselves and our surroundings has been corroded and leaves us vulnerable to pathologically depressive states. This is not an original conjecture, and even the non-scientific community has begun to combat the putrefying consequences of our sybaritic lifestyle by undergoing ‘dopamine fasts’ (Stokel-Walker, 2019). This fad, born out of the busy and stimulating Silicon Valley area, involves abstaining from any pleasurable activities for a given time (including even eye-contact and sex) in order to ‘reboot the brain’. Whilst there are scientific inconsistencies with this rationale, perhaps there is some method to the madness.
To backtrack from this tangential line of discussion, both extrinsic and intrinsic motivation likely hold some degree of influence over personality manifestation. Indeed, Method Teaming’s PIAV (Personal Interests, Attitudes and Values) instrument arguably includes measurements of both types, once more owing to its thoroughness as a tool for detailed personality insight in the business world. In brief, PIAV has identified six prominent motivational orientations amongst individuals, derived from observations from Eduard Spranger’s book, ‘Types of Men’ (1928). PIAV’s six motivations include (Bonnstetter, 1999; Klassen, 2004):
-
Theoretical: a passion to discover, systematise and analyse; a search for knowledge.
-
Utilitarian: a passion to gain return on investment of time, resources and money.
-
Aesthetic: a passion to experience the impressions of the world and achieve form and harmony in life; self-actualisation.
-
Social: a passion to eliminate hate and conflict in the world and to assist others in becoming all they can be.
-
Individualistic: a passion to achieve position and to use that position to affect and influence others.
-
Traditional: a passion to seek out and pursue the higher meaning in life and achieve a system for living.
From these six motivational subtypes, it is important to ascertain those that are more extrinsic or intrinsic in nature, seeing as each has a distinct set of physiological correlates within the brain. Taking the above definitions in isolation, it would seem apparent that the ‘Utilitarian’ and ‘Individualistic’ subtypes possess a more extrinsic character; their motivations revolve around the acquisition of something tangible and external – there is an incentive. The other four, however, appear to describe intrinsic motivation, concerned more with the interest and satisfaction that the individual derives from the activity. Ostensibly, there is a certain morality and virtue concomitant with intrinsic motivation that is not found in extrinsic motivation. For instance, I doubt an individual would be as pleased to be told that they are driven more by external reward rather than inherent satisfaction. Like the football player who performs their utmost so as to lift the trophy and hear the crowd roar their name, or the one who plays for the sheer love of the game. That is not to say there is no worth or quality in extrinsic motivations, for such dispositions are much desired in the professional world. In fact, from an evolutionary perspective, even intrinsic motivations may not be as upstanding as they seem. Under the evolutionary conceptual framework, all aspects of personality have emerged as a result of some benefit that they confer to the individual’s survival and reproductive capabilities. Indeed, it is thought that intrinsically motivating activities help to satisfy basic psychological requirements, such as feelings of competence (being effective) and autonomy (being the causal agent of one’s actions; DeCharms, 1968; Di Domenico and Ryan, 2017). In fact, the aforementioned undermining effect of extrinsic motivation on intrinsic motivation understandably results in an erosion of competence and autonomy sensations (White, 1959; DeCharms, 1968; Deci and Ryan, 1985; Ryan and Deci, 2017). Additionally, intrinsic motivations help to introduce organisms to novel experiences and thus promote the growth of their skillset and adaptive behaviours for the benefit of confronting future uncertain situations with greater competence (Ryan and Deci, 2017). So, there is undoubtedly a personal payoff provided also by intrinsic motivations – they are simply less conspicuous.
There are two angles at which one can describe the neural correlates of intrinsic motivation. The first does so in terms of neurotransmitter systems. The dopaminergic system, which we have already encountered as an integral classifier of DiSC’s four-factor behavioural model, is unsurprisingly implicated also in motivational traits. Fisher et al. (2009) investigated prominent EEG signatures that are specific to (intrinsically) theoretically motivated school children. When engaged in an academic task, intrinsic motivation correlated positively with increased magnitude of the FRN signal, a classic indicator of dopaminergic activity which we discussed earlier. One fascinating fMRI study (Murayama et al., 2010) examined the dopaminergic dynamics present during the undermining effect-mediated dampening of intrinsic motivation. To emulate sensations of intrinsic motivation that are amenable to the practical constrains of neuroimaging experiments, university student subjects were asked to “play a game-like stopwatch task, in which they were asked to press a button within 50ms of the 5s mark” (Murayama et al., 2010). This task was deemed to be challenging and interesting, and thus a reliable stimulator of intrinsic motivation. In the study, two experimental groups of students underwent this game: a reward group which received performance-dependent monetary rewards upon each successful attempt, and a control group which received no rewards at all. In the first scanning session, both groups demonstrated elevated activity of dopaminergic circuitry, namely at the midbrain (of the brainstem) and caudate (part of the dorsal striatum in the cerebrum), when engaged in the game. After a series of iterations in which the reward group was administered money based on their performance, subjects from this group were less likely to voluntarily engage in the game during a free-choice time period, in comparison with the control group. During a second scanning session, this behavioural undermining of intrinsic motivation was seen to be mirrored by a dampening of midbrain and caudate activity in the reward group, whilst the control group’s activity in these regions remained unaffected. This observation is consistent with the idea that dopamine is an important requisite for sustaining intrinsically motivated states.
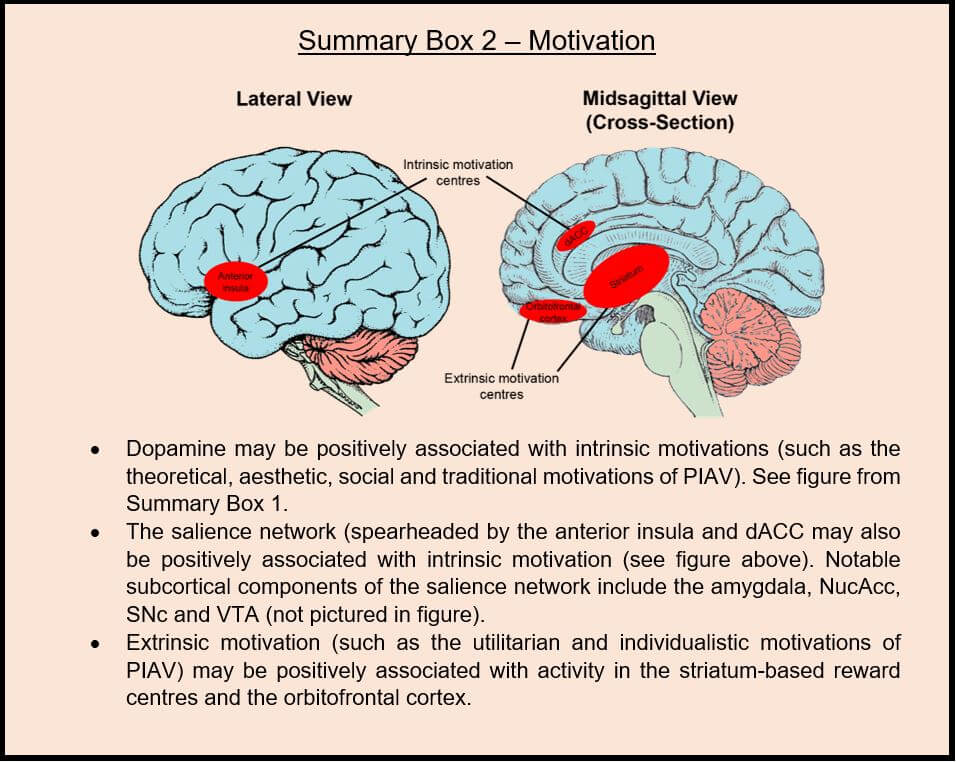
Moving on from the neurotransmitter system perspective, it is also interesting to look at the neural correlates of intrinsic motivation from a large-scale neural network perspective, with a greater emphasis on functional brain region connectivity rather than neurotransmitters. One large-scale neural network significant in effecting intrinsic motivation is the salience network, a collection of functional connections within the brain that aids the recognition of subjectively meaningful events and the engagement of “attentional and working memory resources in the service of goal-directed behaviour” (Bressler and Menon, 2010; Menon and Uddin, 2010; Menon, 2015). The anterior insula and dorsal anterior cingulate cortex (dACC) are the mainstays of this network’s functioning (Di Domenico and Ryan, 2017; Lee and Reeve, 2013, 2017). The anterior insula is an anatomical subsection of the insular cortex, located in the cerebral cortex (the outermost layer of the cerebrum; Craig, 2009). It is responsible for generating those sensations of inherent satisfaction that come with executing an activity that one deems to be subjectively interesting and enjoyable. There is a positive correlation between the magnitude of these sensations and the activity observed from the anterior insula (Lee and Reeve, 2013). The dACC is the dorsal part of the anterior part of the cingulate cortex, located towards the front of the cerebrum, and is implicated in many higher order executive functions (Bush et al., 2002). Some major subcortical regions of the salience network include the amygdala, NucAcc, SNc and VTA (Di Domenico and Ryan, 2017). It is thought that these subcortical nodes send information regarding the motivational significance of stimuli to the anterior insula and dACC, which in turn collate and interpret this information and broadcast a global signal to mobilise attentional and working memory resources for the purpose of engaging in the activity (Di Domenico and Ryan, 2017). Interestingly, there is an evident crossover here with the dopaminergic perspective of intrinsic motivation; the subcortical input to the anterior insula and dACC is often dopaminergic in nature, particularly regarding the SNc and VTA (brainstem initiators of dopamine signalling), NucAcc (a cerebral dopaminergic region) and the amygdala (which receives dopaminergic projections from other dopaminergic regions; Bromberg-Martin et al., 2010; Menon and Uddin, 2010; Menon, 2015). In summary, the encoding of intrinsic motivation appears to operate at two levels – the initial subcortical dopaminergic messengers and the cortical contextualisers and mobilisers.
The neuroscientific literature on extrinsic motivation is surprisingly sparse in comparison. However, some pertinent studies do exist, and show that the physiological correlates of extrinsic motivation are distinct to those of intrinsic motivation. Specifically, relevant neural circuits include the striatum-based reward centre and the orbitofrontal-striatal circuit (Reeve and Lee, 2019). Indeed, as Lee et al. (2012) state: “As people experience extrinsic motivation toward a task, they show greater orbitofrontal cortex activity as they weigh the value of the incentive being offered, and greater anterior cingulate cortex as they go through a pros versus cons decision-making process as to whether engagement in the activity will bring enough benefit to justify the effort expenditure.” As we have previously encountered, the striatum is a dopaminergically-governed reward centre deep within the cerebrum. It is unsurprising that dopamine is also implicated in extrinsic motivation, given its central role in regulating motivation generally. The orbitofrontal cortex localises to the prefrontal cortex, sitting just above the eye sockets, and is heavily complicit in higher order decision making. It is also a major pool of dopaminergic inputs from across the brain (Kringelbach, 2005).
At this juncture, it is beneficial to frame this collection of research back into the context of PIAV’s perspective on human motivation. For instance, it would seem that those circuits and regions that have been implicated in intrinsic motivation are more consistently aroused in individuals who display a stronger theoretical, aesthetic, social or traditional motivational orientation. On the other hand, circuits and regions implicated in extrinsic motivation research are likely to be more consistently activated in those individuals who show a stronger utilitarian or individualistic motivational orientation. Perhaps this is an over-simplification, and there is in fact a co-existence of intrinsic and extrinsic aspects within each of these six subtypes. A more robust set of psychologically-determined definitions of these subtypes might help to match them accurately to their respective neuroscientifically-determined subtypes. However, despite the significance and intrigue this intrinsic-extrinsic research, motivation as a phenomenon clearly extends in its complexity beyond the intrinsic-extrinsic dichotomy, as suggested by PIAV’s six stratifications. Regrettably, I feel it unwise to delve into each of these individually within this review. The breadth of content is too vast to collate and discuss meaningfully, and the depth of content is yet too shallow to afford any confidence in posited hypotheses. A reporting here of the current findings would be superficial at best, and to issue knowledge incompletely is arguably more dangerous than withholding it altogether. Furthermore, as previously mentioned, it is difficult to correlate the psychological definitions of the six subtypes with the neuroscientific terminology. The two disciplines appear to have started their investigations in isolation. Hopefully, as the field develops, greater interdisciplinary communication will foster conceptual and terminological consistencies that bridge unconnected pools of literature into one comparable collection.
4. HVP – The Cognitive Element of Personality.
So far in this review, we have considered the behavioural and motivational elements of personality, in light of Method Teaming’s instruments. We now come to the last element of personality assessed by Method Teaming – cognition. Like much of the terminology encountered thus far, the word ‘cognition’ has been tossed about and overworked so much that, in each instance of its use, its definition as settled by the author must be clearly stated. Thus, for the purpose of this review, I refer to the official definition of the Oxford University Press: “the mental action or process of acquiring knowledge and understanding through thought, experience, and the senses”, which itself is founded on several higher order mental functions such as attention, memory, judgement, reasoning, decision making and problem solving. Clearly, these functions are also somewhat implicated in the behavioural and motivational processes already discussed. For instance, judgement and reasoning (and their respective neural correlates) are engaged when evaluating the prospective value and associated risk of an extrinsically motivating activity. However, cognition extends beyond the regulation of motivational states, and also applies to our belief systems, knowledge, thoughts and ways of thinking. This distinction is better exemplified by observing the output of the HVP (Hartman Value Profile), Method Teaming’s instrument for cognitive assessment. It seeks to encapsulate an individual’s cognitive structure in terms of their value systems and thinking styles. Evidently, this element of personality is far harder to measure than, say, behaviour, which is overtly detectable (Johnson, 2019). Yet HVP has demonstrated astonishing accuracy in ascertaining individuals’ cognitive structures through its carefully constructed self-report assessments, and has been consistently endorsed by the scientific community across the globe (Cone et al., 2012; Byrum et al., 2016; Ruiz, 2017; Nistal-Nuno, 2019).
HVP defines individuals across three dimensions of value: intuitive thinking, pragmatic thinking, and conceptual thinking. Broadly, the intuitive cognitive structure relates to people; it represents the proclivity to effectively empathise, connect and bond with other people as well as oneself. It’s governed by the ‘heart’. The pragmatic thinking style is all about tasks, reflecting the ability to process the environment in real-time when we count, measure, weigh and compare tangible materials. It’s governed by the ‘hand’. Lastly, conceptual thinking is concerned with systems, and is the cognitive architect of rules, order, meaning, plans, and the future in relation to ourselves and the world around us. It’s governed by the ‘head’. It is important to emphasise that each value dimension encapsulates our impressions of both the world and ourselves. For each individual, HVP allocates a level of ‘clarity’ and ‘attention’ in corresponding to the three value dimensions. Clarity is a measure of your natural ability to see and understand the value dimension. On the other hand, attention is a measure of your natural ability to attend to, or pay attention to, specific information to make a decision. This is an extremely vital distinction to make; one’s tendency to orient towards a mode of thinking does not necessarily correlate with the lucidity of that thinking. Consequently, HVP can provide unparalleled insight into an individual’s cognitive structure, surpassing superficial categorisations and going deeper to elucidate the nuances of each dimension.
With the psychological framework built, we can now fortify it with the results from the corresponding neuroscientific research and ask: what are the neural correlates of these cognitive structures? Unfortunately, the literature of this field is sporadic at best. As we have journeyed in this review from behaviour to motivation to cognition, we have simultaneously been stretching the limits of current scientific understanding, and the pool of relevant papers peters out. At this point, it seems as though such deep traits of personality start to split the seams of our articulated consciousness, that does its utmost to explain things rationally through scientific reductionism. Some things, it seems, are inherently more complex and exist beyond the realm of empirical experience – at least for now.
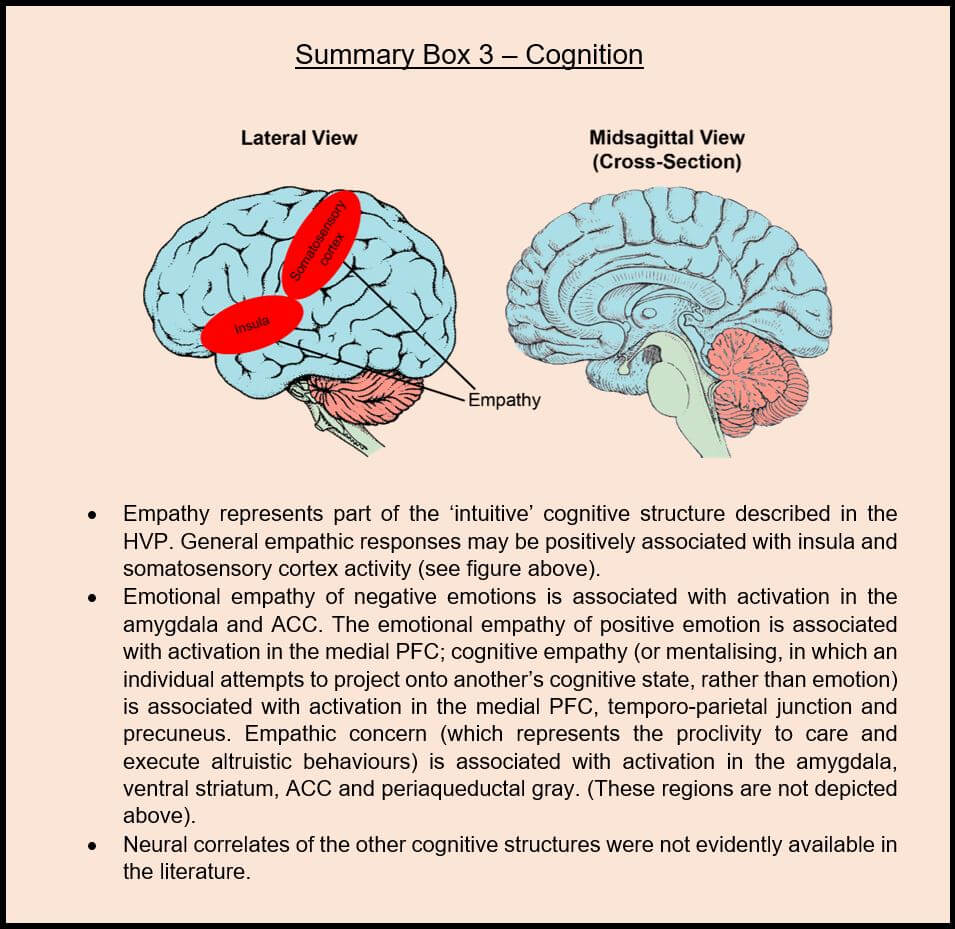
However, upon scouring the online literature databases for relevant primary papers on this topic, there are a few that shine forth. For instance, empathy is a trait analogous to the ‘world’ dimension of the ‘intuitive’ cognitive structure, and has been researched in a neuroscientific context. Empathy represents the “capacity to identify one’s own feelings and needs with those of another person” (Maclean, 1967). Empathic pain is one of the most studied phenomena of this field and has greatly advanced our neural modelling of empathy more generally. For instance, functional neuroimaging research has repeatedly shown that an empathic experience of another’s pain requires mobilisation of the very circuits that are activated when experiencing that pain first-hand (Marsh, 2018). These circuits / regions include the first-order pain processing regions collectively known as the ‘pain matrix’ (Lamm et al., 2011), the second-order pain processing regions (such as the somatosensory cortex, posterior insula and periaqueductal gray) (Zaki et al., 2016), and integrative regions concerned with the motivational and affective facets of pain (such as the ACC and anterior insula) (Corradi-Dell-Acqua et al., 2016). In addition to these correlational studies that are, by definition, limited in their capacity to identify relevant brain regions definitively, a body of causally-driven studies have been able to corroborate the findings. Three studies in particular have executed this effectively, using similar methodologies (Rutgen et al., 2015a; Rutgen et al., 2015b; Rutgen et al., 2018). By administering a placebo analgesic to human study participants prior to a painful electrical stimulation, the subjective measures of pain and the assessments of others’ pain are reduced equivalently. Crucially, these reports are mirrored by an identical dampening of ACC and anterior insula activity in both study groups. This reinforces the credibility of a simulation-based hypothesis for empathy, in which the capacity to empathise with another’s experiential state requires an activation of the same brain regions that are engaged when experiencing the state first-hand.
However, clearly, empathy can assume several other forms than just an empathy of pain. One proposed conceptual framework has stratified empathy into four distinct categories: emotional empathy (negative emotions), emotional empathy (positive emotions), cognitive empathy, and empathic concern (Bird and Viding, 2014). Once more, the simulation-based model assumes that the brain regions responsible for manifesting the first-hand experience of each of these dissociable states are also implicated during the empathic experience of that state. A number of correlational fMRI studies have substantiated this original hypothesis (reviewed in Marsh, 2018), the results of which I will only briefly summarise to avoid unnecessary tangential discussion. Firstly, emotional empathy of negative emotions is associated with activation in the amygdala and ACC; the emotional empathy of positive emotion is associated with activation in the medial PFC; cognitive empathy (or mentalising, in which an individual attempts to project onto another’s cognitive state, rather than emotion) is associated with activation in the medial PFC, temporo-parietal junction and precuneus; and empathic concern (which represents the proclivity to care and execute altruistic behaviours) is associated with activation in the amygdala, ventral striatum, ACC and periaqueductal gray.
From this research, we can confidently assume the validity of the simulation model for empathy. However, it is not obvious to me that these dissociable collections of brain regions are also responsible for instigating the empathic response in the first place. For instance, a greater baseline activity of brain regions governing negative emotion does not necessarily mean that the individual also has a greater capacity / proclivity for empathising with negative emotion in other individuals (although I’m sure that this relationship exists to some degree). Clearly, the negative emotion regions are required to produce an affective empathic sensation that mirrors the sensation of a first-hand experiencer. But the first-hand and empathic responses are ultimately different, so what separates the two neurologically? One fMRI study has posited that activation in the insula and somatosensory cortex are two prominent neural correlates underlying individual differences in empathy generally (Allen et al., 2017). This seems intuitive, given that an empathic response would rely on the ability to interpret the environment (i.e. another individual to whom the empathic response is directed) via sensory perception (governed by the somatosensory cortex) and the ability to integrate this information and instigate an empathic response (likely governed in part by the insula). Consequently, the insula and somatosensory cortex activity are likely important biomarkers of individual differences in general empathy – the ‘world’ dimension of the ‘intuitive’ cognitive structure.
This is all great, but taking the intuitive cognitive structure as a whole, it’s not enough to define it conclusively from the neuroscientific perspective. Firstly, many of these studies are correlational in nature, the limitations of which have already been discussed. Secondly, the current neuroscientific models of empathy do not account for the proven dissociable elements of attention and clarity that are defined in HVP; there is an acknowledgement of the distinction between empathic ‘proclivities’ and ‘capacities’ (Meffert et al., 2013; Seara-Cardoso et al., 2015; Olsson et al., 2016; Zaki, 2016), but this is not similar enough to the attention-clarity dichotomy. Thirdly, neuroscientific literature regarding the ‘self’ dimension of the intuitive value structure, namely self-esteem, is lacking. Without this, we cannot provide an exhaustive account of the intuitive cognitive structure.
As for the remaining cognitive structures – the practical and conceptual value systems – the neuroscientific literature is absent or too primitive to report meaningfully. After an exhaustive search of the online databases, only a smattering of primary papers was returned. It remains possible that some relevant research has been conducted under a different terminological framework that evaded the search terms, but this is quite unlikely. Despite this paucity of knowledge, I do not think it rash to assume that the manifestation of these other cognitive structures is principally grounded in the cerebral cortex, especially in view of the cortically-dominant results of the before-mentioned empathy research. In summary, decoding the neural basis of cognition is complex. HVP provides a robust psychological definition and stratification of cognitive structure. It remains now for neuroscientists to harness this conceptual framework and dispense their entire arsenal of methodologies until something gives way. That is the hope, at least.
-
Conclusion.
The cracks in personality’s inscrutable façade have begun to show. However, I do not necessarily state that triumphantly. Iain McGilchrist, renowned psychiatrist and literary scholar, often writes of the unnatural dominance of our left hemispheric processing that has arisen in modern-day society – processing dictated by a narrow, focused attention that has a compulsion to categorise and characterise (McGilchrist, 2009; McGilchrist 2010). Perhaps we have forgotten the essentiality of the intuited and the implicit through our contemporary proclivity to provide the explicit. Perhaps personality requires a certain incoherence, for by defining it we lose its intrinsic value. Or perhaps not. I do not pretend to know, and nor should anyone else. Not at this early stage of our understanding.
And yet, there is a clear benefit to understanding ourselves and those around us with greater clarity. OND’s Method Teaming tool provides the means with which this can be achieved. For all the transcendent and mysterious qualities of personality, we have seen that Method Teaming is grounded in the psychological science and substantiated by the neuro-science. Therefore, neuroscience will remain indispensable for continuing personality research in the future. At this stage, the research approach concerns identifying neural correlates – those brain regions and components that show activity when a particular element of personality is being engaged, and are thus proposed as candidates for its causation. After many repetitions of these experiments, across different imaging modalities and personality elements, we can start to build an atlas of personality as seen through the neuroscientific lens. This atlas is by no means complete, but is well on its way.
I wrote at the start of this review that Method Teaming’s credibility would not be tarnished if it is discovered that its instruments do not map onto every single region of the brain. Indeed, we have learnt that some regions, namely the cortical regions of the cerebrum, contribute more to personality traits than other regions, such as the cerebellum and brainstem. What is important here is that the instruments map to distinct parts of the brain and are governed by distinct circuits. In other words, the comprehensiveness of Method Teaming’s psychological foundation has been echoed by the neuroscience. The instruments do not map repeatedly to the same part of the brain as some instruments are believed to do, minimising their overlap and redundancy as they reveal more of the mind’s secrets. Unlike others in its field, Method Teaming caters for three aspects of personality: behaviour, motivation and cognition. Not only does it report these aspects in isolation, but also goes a step further to integrate each of their outputs into an overall classification of personality: Networker, PD, Strategist, and EQ. In this way, the holistic nature of personality is not lost amongst the reductionism that is necessary to decode it. When Method Teaming shines a light on the personality of individuals, its comprehensiveness means the illumination it gives is truly broad and full. Businesses and organisations can have confidence in this: the neuroscience speaks, and it says that the method behind Method Teaming is robust and dependable.
-
References.

